Draw as Many Unique Lewis Structures as Possible for C4h10.
How many structural isomers are possible for the molecular formula C4H10 quizlet. 5 Which type of isomerism is possible in C4H10 butane.

Draw Two Lewis Structures For A Compound With The Formula C4h10 No Atom Bears A Charge And All Brainly Com
Anyanavicka 17 9 months ago.

. A step-by-step explanation of how to draw the C4H10 Lewis Dot Structure ButaneFor the C4H10 structure use the periodic table to find the total number of v. 6 What is the structural formula for C4H10. 7 How many structural isomers are possible.
Because each Carbon needs to. List truths about constitutional isomers. How many structural isomers of C4H10 are possible.
Draw two lewis structures for a compound with the formula c4h10. 3 How many structural isomers are possible for the molecular formula C4H10 quizlet. Do not consider cyclic ring structures.
As a general rule when several lewis structures are possible the most stable one will be that in which 1 the atoms bear the smallest formal electronegativity differences between atoms are important in determining the actual charge distributions in. They have the same. Draw two Lewis structures for a compound with the formula Draw two Lewis structures for a compound with the formula C4H10.
For Butane we have a total of 26 valence electrons. There are 2 possible isomers of C4H10. Use your diagram to answer the following questions.
Below is the figure attached. R 0082 L atm K1 mol1 - 21174558. Draw two lewis structures for a compound with the formula c4h10.
4 How many structural isomers can you draw having molecular formula c3h4o. At what temperature would 210 moles of Nitrogen gas have a pressure of 125 atm and in a 250 L tank. We have to show both.
That makes it a little bit easier to draw the C4H10 Lewis structure. Answer 1 of 2. 2 Lewis Structures For C4H10No need for the electron dots because the hydrogen bonds completed the octet rule.
There is more than one acceptable diagram with the same answers. Whenever we see the ending ane we know that were going to have Carbons and Hydrogens single bonded. 1 on a question Draw as many unique lewis structures as possible for c4h10.
No atom bears a charge and all carbons have complete. How many structural isomers are possible for a substance having the molecular formula C4H10. The number of C-H bonds The number of C-C single bonds The number of CC double bonds The total number of lone pairs Submit.
There are only two isomers for C4H10. It has two isomers. Here is the trick to find isomersstructural only Just find out degree of unsaturation DOU By this formula- DOU C1 - HN2 where C.
Solution for Draw Lewis structures fora two compounds of formula C4H10 b two compounds of formula C2H6O. No atom bears a charge and all carbon atoms have complete octe. Solved Expert Answer to Draw the Lewis structure for butane C4H10 given the structure contains four carbon atoms bonded in a row.
Related Question Answers Draw chain and ring structures of organic compound having six carbon atoms in it. No atom bears a charge and all carbon atoms have complete octets. All the carbon and hydrogen has complete octet.
Well put four Carbons in a row and then well put Hydrogens around them. Draw a Lewis structure for C4H10. Constitutional isomers are chemical.
Butane is an alkane with four carbon atoms so molecular formula is C 4 H 10. This is the C4H10 Lewis structure. 2 How many structural isomers of C4H10 are possible.
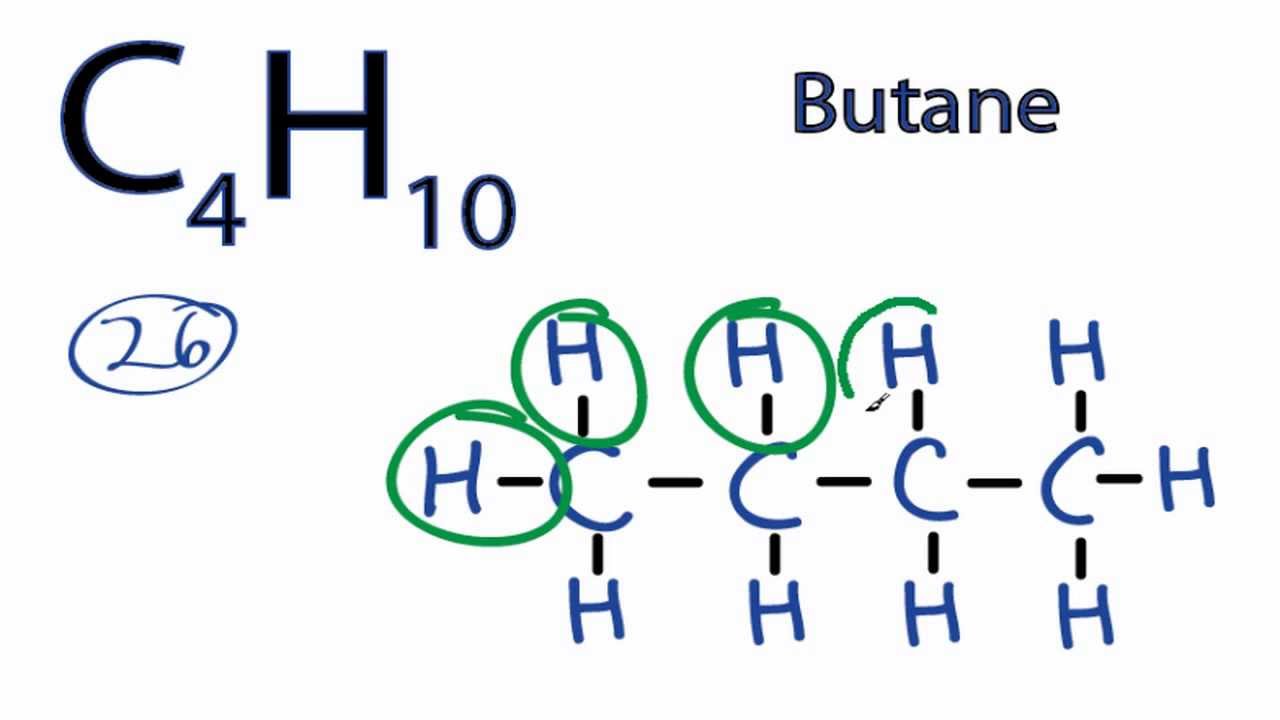
C4h10 Lewis Structure How To Draw The Lewis Structure For C4h10 Youtube

What Are The Possible Isomerisms Of C4h10 Quora

Draw Two Lewis Structures For A Compound With The Formula C4h10 No Atom Bears A Charge And All Brainly Com
Comments
Post a Comment